Scientists from the McGovern Institute for Brain Research of the MIT and the Broad Institute of Mit and Harvard have renovated a compact enzyme guided by RNA they found in bacteria in an effective and programmable publisher of human DNA.
The protein they have created, called Novaïscb, can be adapted to make specific modifications to the genetic code, modulate the activity of specific genes or perform other editing tasks. Since its small size simplifies delivery to cells, Novaisc developers say that it is a promising candidate for developing gene therapies to treat or prevent disease.
The study was led by Feng ZhangThe professor of neuroscience of James and Patricia Poitras at MIT who is also an investigator of the McGovern Institute and the Howard Hughes Medical Institute, and a basic member of the Broad Institute. Zhang and his team reported their work to free access this month in the newspaper Nature Biotechnology.
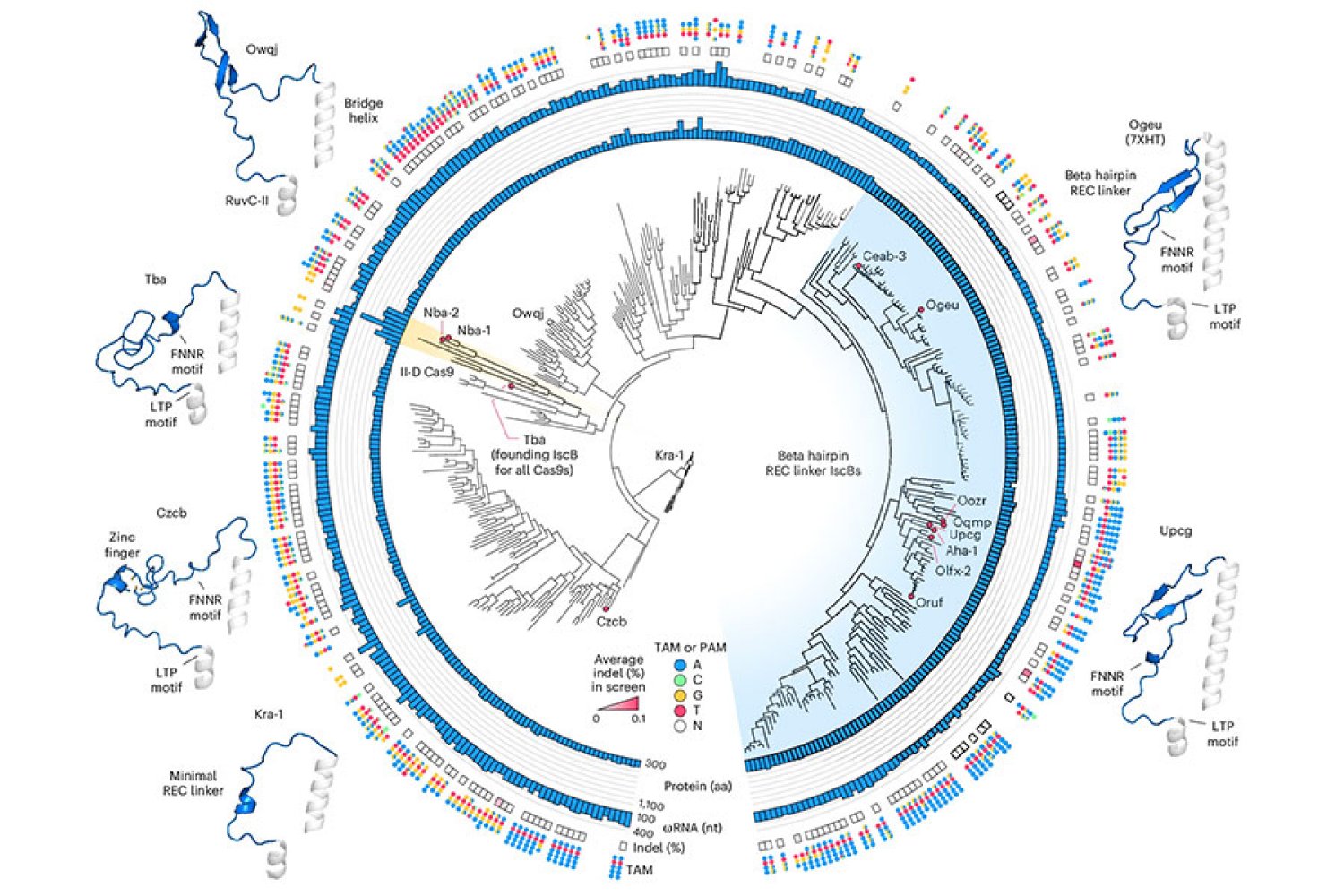
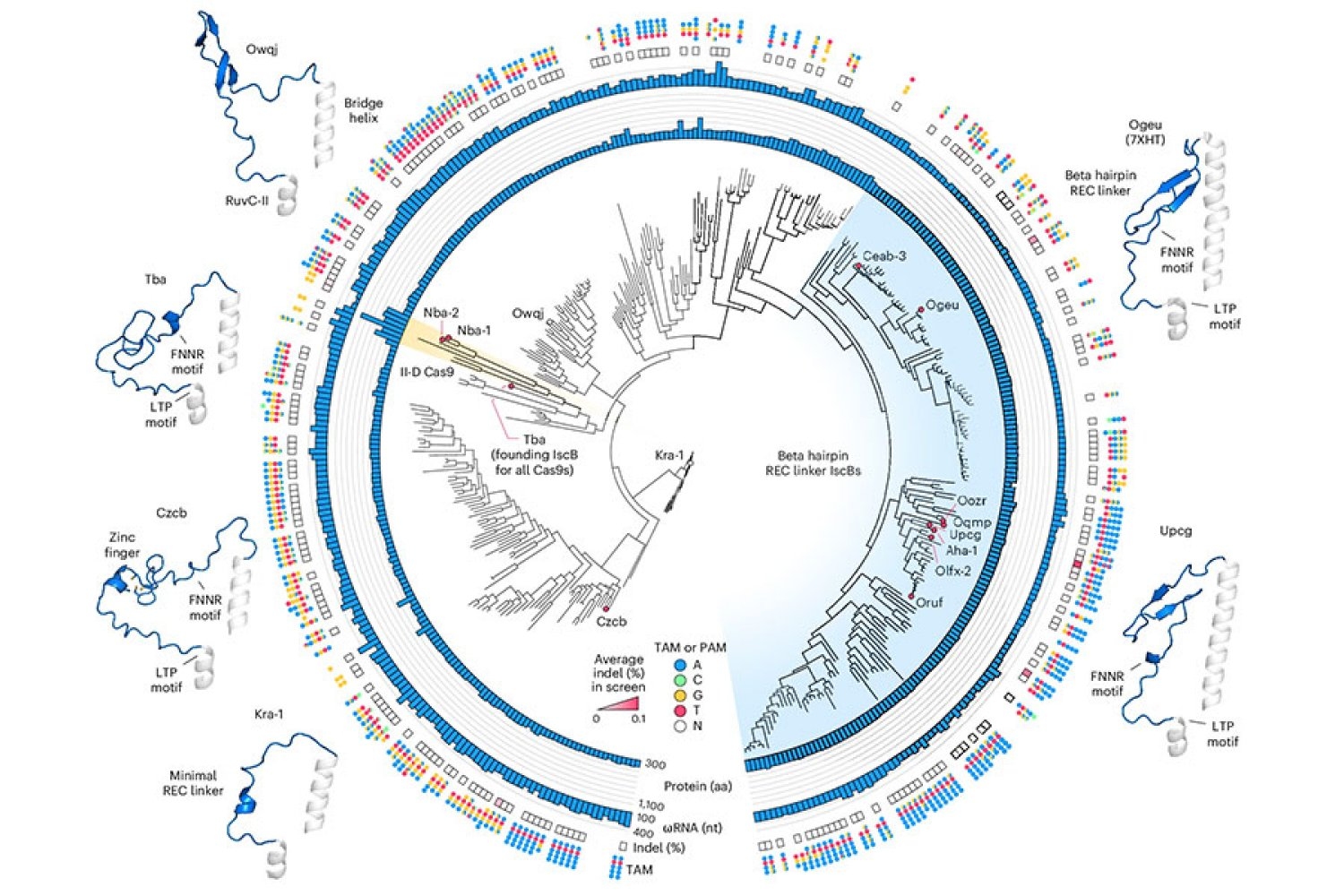
Novaïscb is derived from a bacterial CUT CUT which belongs to a family of proteins called ISCBS, which the Zhang laboratory discovered in 2021. ISCB are a type of omega system, the evolutionary ancestors of CAS9, which is part of the CRISPR bacterial system and Zhang and others have transformed into powerful genome tools. Like CAS9, ISCB enzymes cut DNA on the sites specified by an RNA guide. By reprogramming this guide, researchers can redirect enzymes to target sequences of their choice.
ISCBS had drawn the attention of the team not only because they share the key characteristics of the CRISPR DNA Cup9, but also because they are a third of its size. It would be an advantage for potential gene therapies: compact tools are easier to provide cells, and with a small enzyme, researchers would have more flexibility to tinker, potentially adding new features without creating too large tools for clinical use.
According to their initial studies on ISCB, researchers from the Zhang laboratory knew that some family members could cut DNA targets in human cells. However, none of the bacterial proteins worked well enough to be deployed therapeutically: the team should modify an ISCB to ensure that it could effectively modify targets in human cells without disturbing the rest of the genome.
To start this engineering process, Soumya Kannan, a student graduated from the Zhang laboratory, who is now a junior at the Harvard Society of Fellows, and Shiyou Zhu Postdoct. They have tested nearly 400 different ISCB enzymes which can be found in bacteria. Ten were able to edit DNA in human cells.
Even the most active of them should be improved to make it a useful genome editing tool. The challenge would be to increase the activity of the enzyme, but only to the sequences specified by its RNA guide. If the enzyme became more active, but without discrimination, it would reduce DNA in involuntary places. “The key is to balance the improvement of activity and specificity at the same time,” explains Zhu.
Zhu notes that the bacterial ISCB are directed to their target sequences by relatively short RNA guides, which makes the restriction of enzyme activity difficult to a specific part of the genome. If an ISCB could be designed to accommodate a longer guide, it would be less likely to act on sequences beyond its planned objective.
To optimize the ISCB for the edition of the human genome, the team has exploited information that the student graduated Han Altae-Tran, who is now postdoctoral at the University of Washington, had learned the diversity of bacterial ISCB and how they have evolved. For example, the researchers noted that the ISCB who operated in human cells included a segment that they called Rec, which was absent in other ISCBS. They suspected that the enzyme might need this segment to interact with DNA in human cells. When they examined the region more closely, structural modeling suggested that by slightly exploiting part of the protein, REC could also allow ISCB to recognize longer RNA guides.
Based on these observations, the team experienced the exchange in certain parts of the REV domains from different ISCBS and CAS9, assessing how each change has had an impact on the protein function. Guided by their understanding of the way in which ISCBS and Cas9 interact with DNA and their RNA guides, researchers have made additional changes, aimed at optimizing both efficiency and specificity.
In the end, they generated a protein they called Novaïscb, which was more than 100 times more active in human cells than the ISCB with which they had started, and which had demonstrated a good specificity for its targets.
Kannan and Zhu built and projected hundreds of new ISCBs before arriving at Novaïscb – and each change they brought to the original protein was strategic. Their efforts were guided by the knowledge of their team of the natural evolution of ISCBS, as well as by the predictions of the impact of each alteration on the structure of the protein, made using an artificial intelligence tool called alphafold2. Compared to traditional methods of introducing random protein changes and screening for their effects, this rational engineering approach has considerably accelerated the team's ability to identify a protein with the characteristics they sought.
The team has shown that NovaïSCB is a good scaffolding for a variety of genome editing tools. “It works biochemically in a very similar way to CAS9, which facilitates the portage of tools that have already been optimized with the CAS9 scaffolding,” explains Kannan. With different modifications, the researchers used NovaïSCB to replace specific letters from the DNA code in human cells and to modify the activity of targeted genes.
Above all, the tools based on NOVISC are compact enough to be easily wrapped in a single adeno -associated virus (AAV) – the vector most often used to provide gene therapy to patients. Because they are larger, the tools developed using CAS9 may require a more complicated delivery strategy.
Demonstrating the therapeutic novaisb potential, Zhang's team has created a tool called Omegaoff which adds chemical markers to DNA to compose the activity of specific genes. They programmed omegaoff to suppress a gene involved in cholesterol regulation, then used AAV to deliver the system to the flashes of mice, resulting in lasting reductions in cholesterol in the blood of animals.
The team expects Novaïscb to be used to target the genome editing tools to most human genes, and I can't wait to see how other laboratories deploy new technology. They also hope that others will adopt their approach guided by the evolution of rational protein engineering. “Nature has such diversity and its systems have different advantages and disadvantages,” explains Zhu. “By learning this natural diversity, we can make systems that we try better and better engineering.”
This study was funded, in part, by K. Lisa Yang and Hock E. Tan Center for Molecular Therapeutics at MIT, Broad Institute Programmable Therapeutics Gift Donors, Pershing Square Foundation, William Ackman, Neri Oxman, The Phillips Family, and J. and P. Poitras.