In order to produce effective targeted therapies for cancer, scientists must isolate the genetic and phenotypic characteristics of cancer cells, inside and through different tumors, because these differences have an impact on the way tumors react to treatment.
Part of this work requires a deep understanding of RNA or protein molecules that each cancer cell expresses, where it is located in the tumor, and what it looks like a microscope.
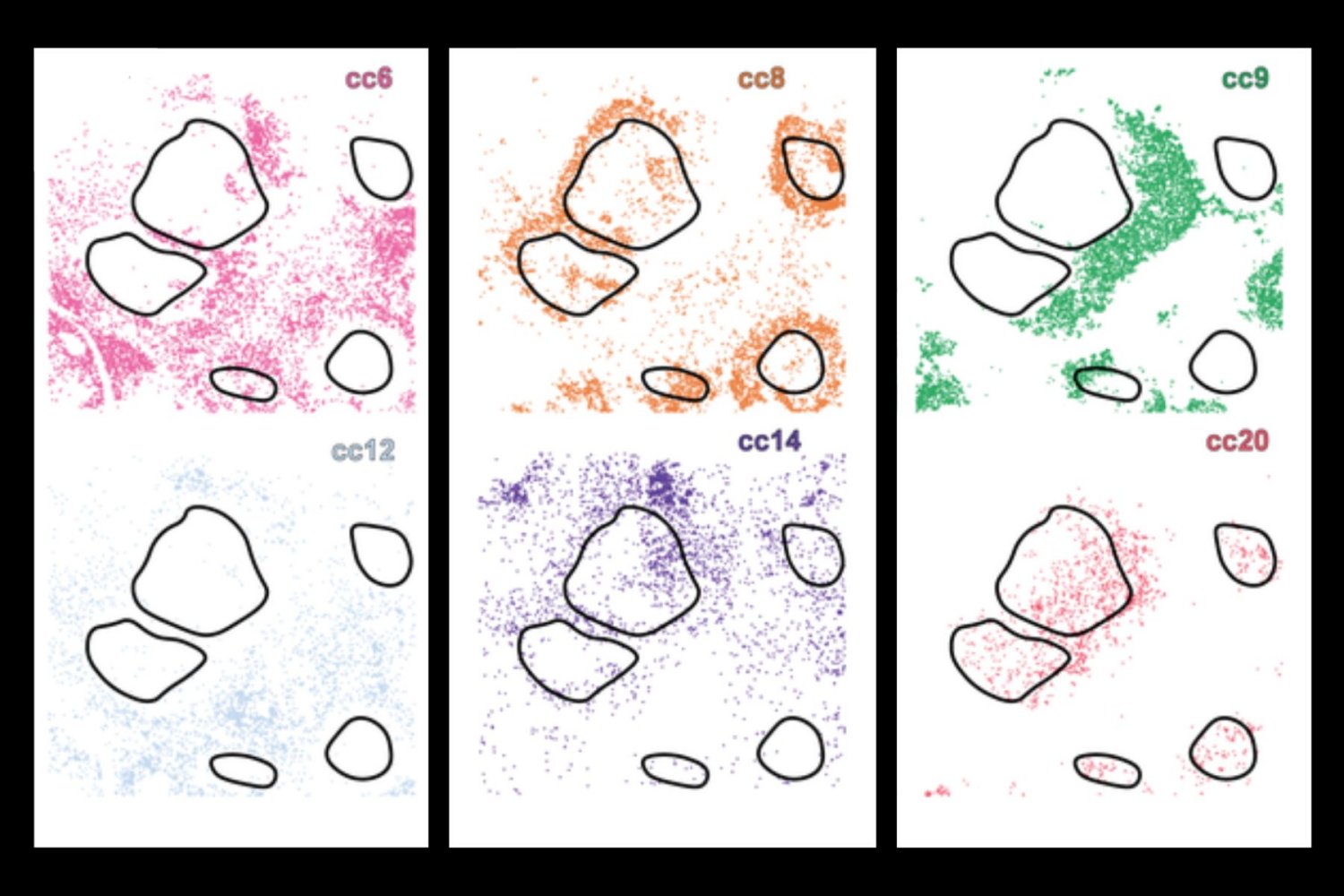
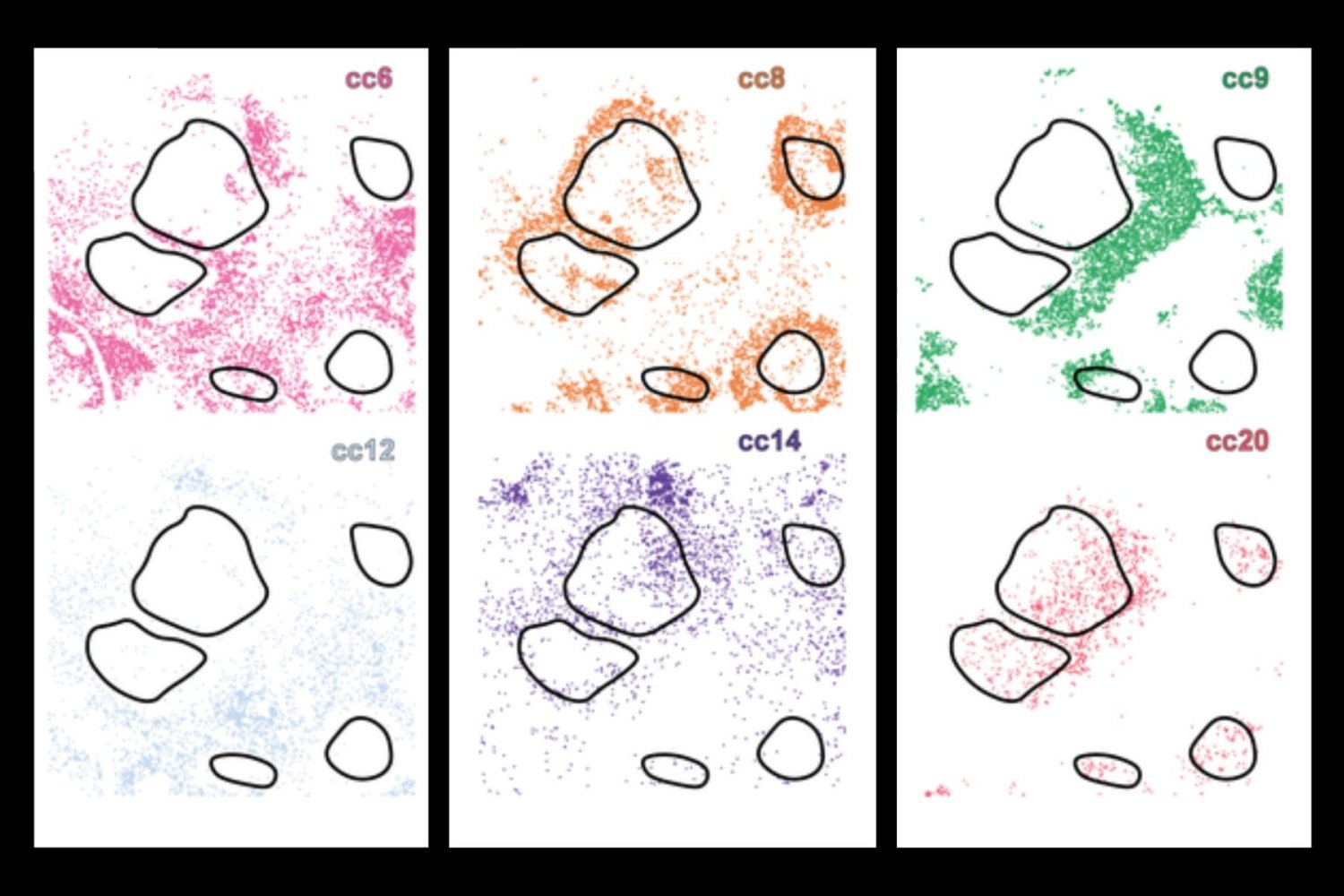
Traditionally, scientists have examined one or more of these aspects separately, but now a new IA tool for in -depth learning, cells (local cellular environment and neighborhood scan), merges the three fields together, using a combination of convolutional neural networks and graphic neural networks to build a full digital profile for each single cell. This allows the system to group cells with a similar biology – effectively separating even those which seem very similar in isolation, but behave differently depending on their environment.
Study, recently published in Nature immunologyDetails The results of a collaboration between MIT researchers, the Harvard Medical School, the University of Yale, the University of Stanford and the University of Pennsylvania – an effort led by Bokai Zhu, a post -doc of MIT and member of the Broad Institute of Mit and Harvard and the Ragon Institute of MGH, MIT and Harvard.
Zhu explains the impact of this new tool: “At the start, we would say, oh, I found a cell. This is called a T cell
“I can use the existing information to better define what a cell is, what is the subpopulation of this cell, what this cell does and what is the potential functional reading of this cell. This method can be used to identify a new biomarker, which provides specific and detailed information on sick cells, allowing more targeted development of therapy. ”
This is a critical advance because current methodologies often lack critical molecular or contextual information – for example, immunotherapy can target cells that only exist at the limit of a tumor, limiting efficiency. Using in -depth learning, researchers can detect many different layers of information with cells, including morphology and where the cell is spatial in a tissue.
When applied to samples of healthy tissue and several types of cancer, including cancer of lymphoma and liver, cells have revealed rare immune cell subtypes and revealed how their activity and location are linked to pathological processes – such as tumor infiltration or immune deletion.
These discoveries could help scientists better understand how the immune system interacts with tumors and pave the way for diagnostics and more precise cancer immunotherapy.
“I am extremely excited by the potential of new AI tools, such as cells, to help us understand more holistically aberrant cellular behavior in tissues,” explains the co-author Alex K. Shalekthe director of Institute of Medical Engineering and Science (Imes), Professor JW KIECKHERFER in Imes and chemistry, and an extra-uros member of the Koch Institute for Integrative Cancer Research at MITas well as a member of the Broad Institute Institute and a member of the Ragon Institute. “We can now measure an enormous amount of information on individual cells and their tissue contexts with advanced multi-ordination tests. Get effectively that data to name new therapeutic tracks are an essential step in the development of improved intervention. When coupled with the right entry data and careful downstream validations, these tools promise to accelerate our positive impact capacity for human health and welcome. ”. ».